Preclinical Toxicology - Vivisciences Preclinical CRO
Preclinical Toxicology
Ensuring Safety Through Rigorous In Vivo Science
Setting New Standards in Drug and Device Evaluation through Rigorous Toxicology

Comprehensive Studies
Wide range of preclinical toxicology tests. Tailored to assess the safety of your products.

Regulatory Compliance
Aligned with global safety guidelines. Ensures smooth and successful submissions.

Expert Support
Guidance through every study phase. Partnering for reliable, accurate results.


Edit Content
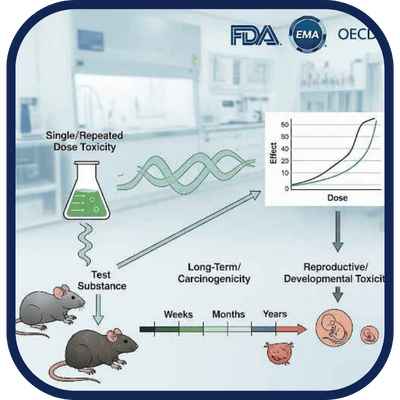
We conduct regulatory-compliant in vivo studies to characterize systemic and long-term toxicity.
Animal studies that assess toxic effects following single or repeated doses, including long-term
(carcinogenicity), reproductive, and developmental toxicity evaluations. Required by global health
authorities (FDA, EMA, OECD) for pharmaceuticals, APIs, and in certain cases for food additives
and medical devices to demonstrate product safety.
Edit Content
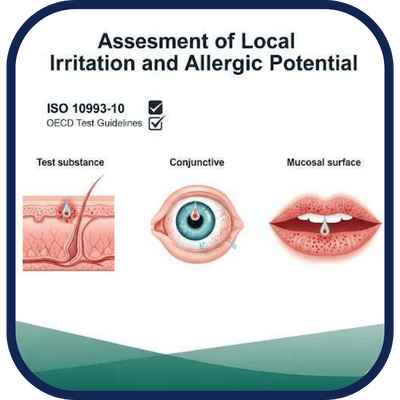
We assess local irritation and allergic potential for topically applied or contact-based products.
Evaluation of the potential of a substance to cause irritation or allergic reactions at the site of contact
such as skin, eyes, or mucosal surfaces. Essential for topical pharmaceuticals, cosmetics, and medical
devices to meet ISO 10993-10 and OECD test guidelines.
Edit Content
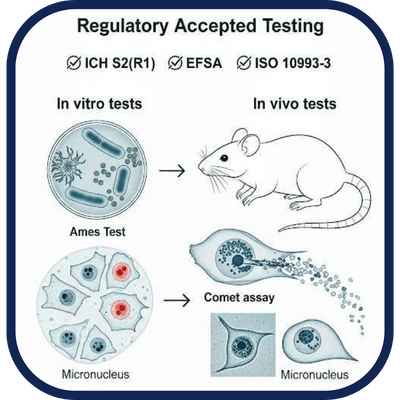
We perform standard and regulatory-accepted genotoxicity testing to detect DNA damage.
In vitro and in vivo tests (e.g., Ames, micronucleus, comet assay) to assess a compound’s potential to
cause genetic mutations or chromosomal damage. Mandatory for new pharmaceuticals, APIs, and
relevant food additives and medical devices under ICH S2(R1), EFSA, and ISO 10993-3.
Edit Content
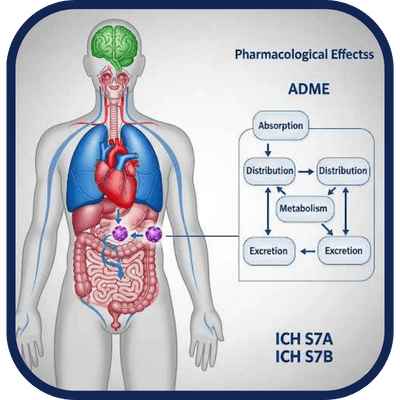
We provide insight into the pharmacological effects and systemic disposition of test compounds.
Evaluation of a substance’s effects on vital organ systems (cardiovascular, respiratory, CNS) and its
absorption, distribution, metabolism, and excretion (ADME). Required for pharmaceutical
development under ICH S7A/B and critical to support human risk assessments and dose setting.
Edit Content
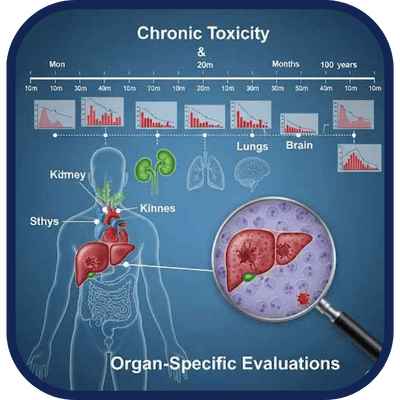
Chronic Toxicity & Organ-Specific Evaluations
We evaluate long-term systemic effects and organ-specific toxicity through tailored in vivo protocols.
Long-duration toxicity studies designed to detect cumulative effects or target organ toxicity after
repeated exposure Essential for chronic use pharmaceuticals, implantable medical devices, and
compounds with prolonged exposure profiles.
Edit Content
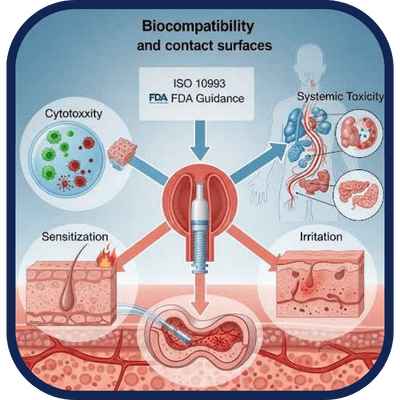
We assess material safety and biological response for devices and contact surfaces.
A battery of tests evaluating cytotoxicity, sensitization, irritation, and systemic toxicity to ensure
compatibility of materials with biological systems. Required for medical device regulatory
submissions under ISO 10993 standards and FDA biocompatibility guidance.
10
+
Years Experience
395
+
Projects Done
50
+
Portfolios
Vivisciences Preclinical CRO – Advancing Drug Safety and Development
- Comprehensive Preclinical Services: Vivisciences offers GLP-compliant toxicology, pharmacology, and safety studies for pharmaceuticals and medical devices.
- Expert CRO Partner: Trusted contract research organization with deep expertise in preclinical toxicology and regulatory strategy.
- Regulatory-Ready Reports: Delivering high-quality data and study reports to meet global regulatory requirements (FDA, EMA, etc.).
- In Vivo Excellence: Specializing in in vivo toxicology studies to assess safety, tolerability, and risk before clinical trials.
- Customized Study Designs: Tailored toxicology programs aligned with your compound’s development timeline and therapeutic goals.

Plan to Start a Project


