Computational (In Silico) Toxicology : ICH M7 & Q3D Guidelines
Transforming Toxicology with Predictive Precision
Expert in Predictive Modeling , Cheminformatics & Risk Assessments for Chemical Safety Evaluation.

Transforming Toxicology
Advancements in computational science enable faster, cost-effective chemical safety evaluation.

VIVISCIENCES Services
Comprehensive in silico and predictive modeling solutions for diverse industries.

Supporting Compliance
Helping regulatory risk assessment across pharmaceuticals, cosmetics, chemicals, and devices.


Predictive Computational Approaches Revolutionizing Toxicity Assessment
Computational methods that predict the toxic potential of a substance based on its chemical structure.
QSAR (Quantitative Structure-Activity Relationship): Uses mathematical models to link chemical structure with biological activity
Read-Across: Predicts toxicity by comparing to similar compounds with known data.
TTC (Threshold of Toxicological Concern): Establishes exposure limits for chemicals with limited data based on structural alerts. Regulatory-driven hazard assessment made efficient. Essential for reducing animal testing while supporting REACH, ICH, and FDA submissions through validated predictive models.
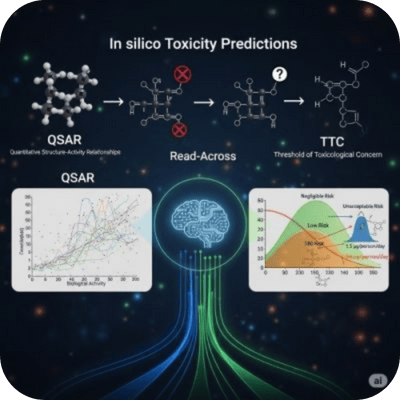
Accelerating Discovery: AI-Driven Validation & Virtual Screening for Safer, Smarter Chemicals
Use of software tools to validate and screen large sets of chemical compounds for toxicity, pharmacokinetics, or target interaction based on predictive algorithms.
Speeds up early-stage R&D, reduces lab costs, and supports submission of validated toxicity data in drug, cosmetic, and chemical safety evaluations.
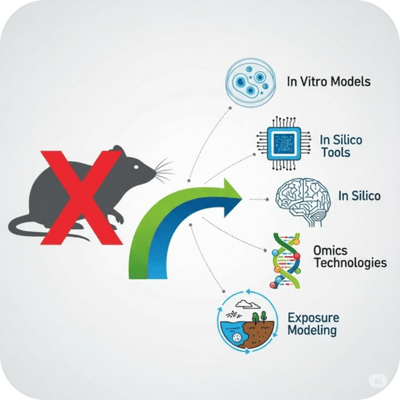
Revolutionizing Safety Testing: Embracing New Approach Methodologies (NAMs)
A collective term for non-animal, modern testing strategies such as in vitro models, in silico tools, omics technologies, and exposure modeling.
NAMs are encouraged or required by the EU Chemical Strategy for Sustainability, OECD, and U.S. EPA to replace animal testing, especially in cosmetics and industrial chemicals.
Safeguarding Medicine
Assessment of DNA-reactive impurities in drug substances/products using two complementary in silico methods (rule-based and statistical), backed by expert review.
Mandatory under ICH M7 (R1) for pharmaceuticals to ensure safety of trace-level impurities over a drug’s lifecycle.

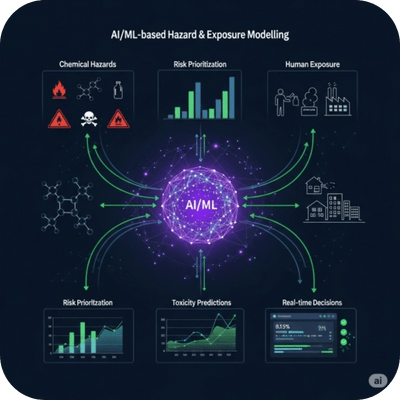
AI-Driven Hazard & Exposure Modeling
Application of artificial intelligence (AI) and machine learning (ML) to predict chemical hazards and human exposure potential using large datasets and trained algorithms.
Supports risk prioritization, toxicity predictions, and real-time decision making in pharma, food, and cosmetics safety.
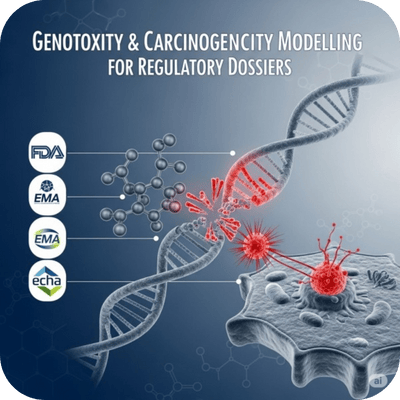
Predictive Genotoxicity Models
In silico models that assess whether a substance can cause genetic mutations (genotoxicity) or cancer (carcinogenicity) through mechanisms like DNA damage or oxidative stress.
Required for regulatory dossiers (FDA, EMA, ECHA) and early safety screening in pharmaceuticals and personal care products.
Process Impurity Safety Shield
Scientific evaluations using TTC, in silico prediction, and risk-based approaches to justify the safety of process-related impurities in drug manufacturing.
Necessary for API and drug product filings under ICH Q3A/B and M7 to demonstrate control of mutagenic impurities.

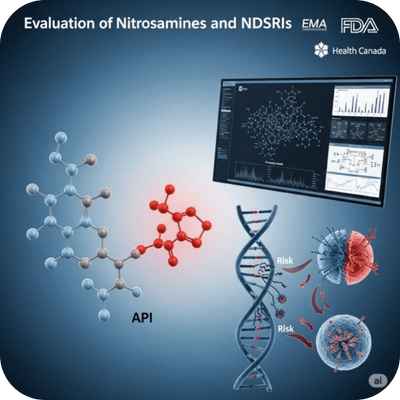
Nitrosamine Risk Evaluation
Assessment of potential nitrosamine impurities and NDSRIs (Nitrosamine Drug Substance Related Impurities) for their carcinogenic risk using predictive models and TTC.
Required globally (EMA, FDA, Health Canada) following detection of nitrosamines in many APIs since 2018.
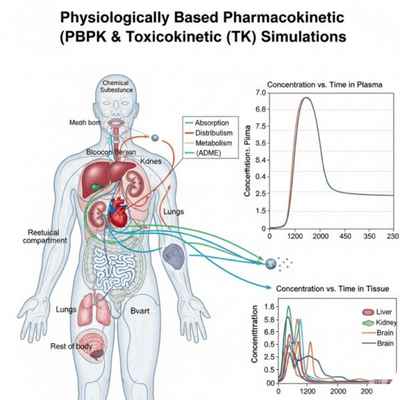
Dynamic PBPK & TK Modeling
PBPK (Physiologically Based Pharmacokinetic) and TK (Toxicokinetic) modeling simulate how chemicals are absorbed, distributed, metabolized, and excreted in the human body.
Supports bioequivalence, dose extrapolation, and risk assessment, especially in medical devices, cosmetics, and special populations (pediatrics, geriatrics).
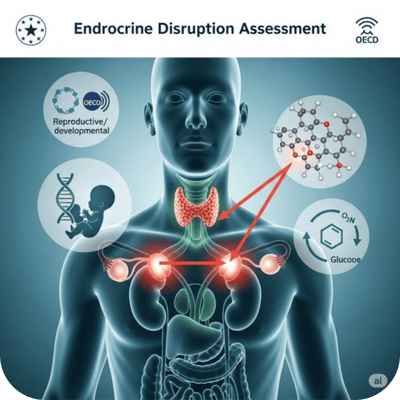
Hormone System Safety Check
Assessment of whether a substance interferes with the hormonal system, potentially affecting reproduction, development, or metabolism.
Required for cosmetics, biocides, REACH registrations, and pesticide approvals under EU and OECD frameworks.
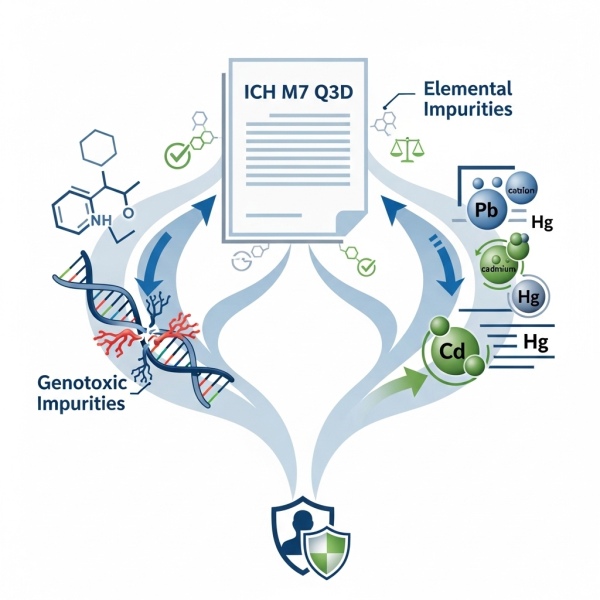
ICH M7 & Q3D Guidelines
- Ensure pharmaceutical compliance with ICH M7 Q3D Guidelines for the safe assessment of genotoxic and elemental impurities.
- Leverage advanced in silico toxicology and risk assessment tools to meet global regulatory standards.
- ICH M7 & Q3D Guidelines protect patient safety by controlling impurities in drug substances and products.
- Trust expert support to navigate ICH M7 Q3D Guidelines and safeguard product quality and patient health.
- Align your pharmaceutical development with ICH M7 Q3D requirements for successful regulatory submissions.